How To Find Osmolality Of Solution . to calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. osmolarity is a measure of the total concentration of solute particles in a solution, influencing the movement of. Osmolarity is the number of osmoles of solute per litre of solution. The osmolarity of solutions containing a single type of solute (for example: Convert that number to osmoles per liter and add. the osmolality of a real solution corresponds to the molality of an ideal solution containing nondissociating solutes and is expressed. how do you calculate osmolarity? you multiply the molarity by the number of osmoles that each solute produces. An osmole is 1 mol of. in simpler terms, osmolality is an expression of solute osmotic concentration per mass of solvent, whereas osmolarity is per volume.
from www.chegg.com
The osmolarity of solutions containing a single type of solute (for example: in simpler terms, osmolality is an expression of solute osmotic concentration per mass of solvent, whereas osmolarity is per volume. osmolarity is a measure of the total concentration of solute particles in a solution, influencing the movement of. to calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. An osmole is 1 mol of. you multiply the molarity by the number of osmoles that each solute produces. how do you calculate osmolarity? Osmolarity is the number of osmoles of solute per litre of solution. the osmolality of a real solution corresponds to the molality of an ideal solution containing nondissociating solutes and is expressed. Convert that number to osmoles per liter and add.
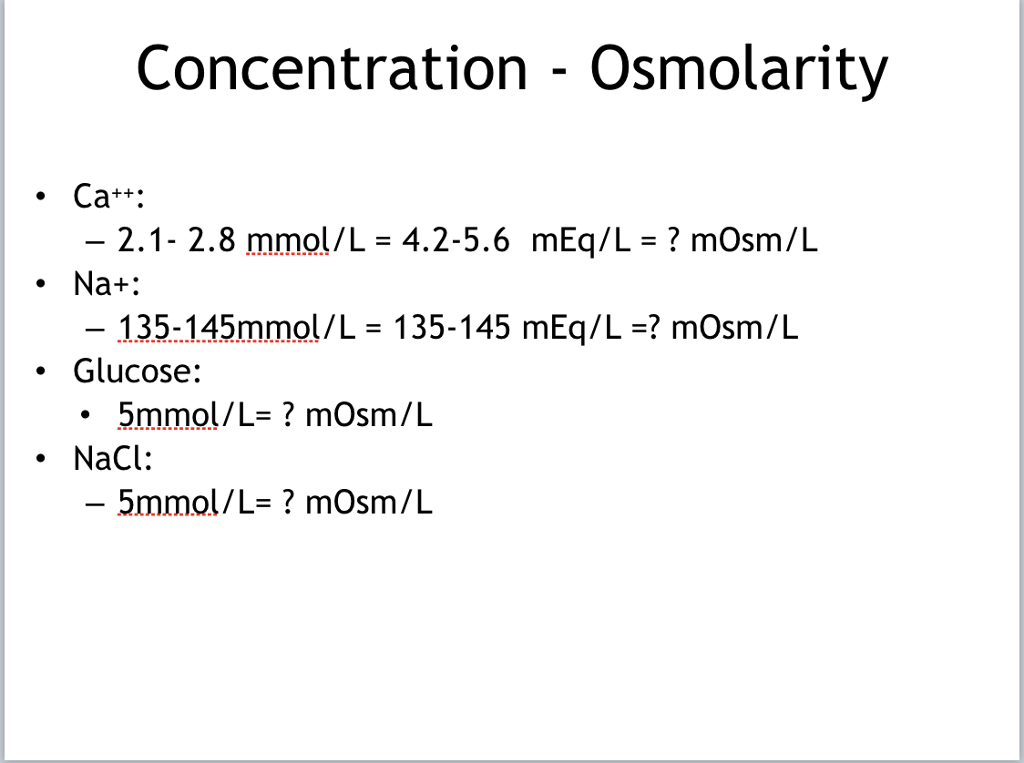
Solved Concentration Osmolarity . Ca 2.12.8
How To Find Osmolality Of Solution An osmole is 1 mol of. the osmolality of a real solution corresponds to the molality of an ideal solution containing nondissociating solutes and is expressed. in simpler terms, osmolality is an expression of solute osmotic concentration per mass of solvent, whereas osmolarity is per volume. An osmole is 1 mol of. osmolarity is a measure of the total concentration of solute particles in a solution, influencing the movement of. Osmolarity is the number of osmoles of solute per litre of solution. The osmolarity of solutions containing a single type of solute (for example: how do you calculate osmolarity? to calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. you multiply the molarity by the number of osmoles that each solute produces. Convert that number to osmoles per liter and add.
From www.slideserve.com
PPT Water Metabolism PowerPoint Presentation, free download ID4493121 How To Find Osmolality Of Solution Osmolarity is the number of osmoles of solute per litre of solution. Convert that number to osmoles per liter and add. in simpler terms, osmolality is an expression of solute osmotic concentration per mass of solvent, whereas osmolarity is per volume. how do you calculate osmolarity? An osmole is 1 mol of. the osmolality of a real. How To Find Osmolality Of Solution.
From www.youtube.com
How to solve osmolarity calculation problems 2 YouTube How To Find Osmolality Of Solution osmolarity is a measure of the total concentration of solute particles in a solution, influencing the movement of. Convert that number to osmoles per liter and add. how do you calculate osmolarity? Osmolarity is the number of osmoles of solute per litre of solution. to calculate the osmolarity of a solution, the first step is to convert. How To Find Osmolality Of Solution.
From ar.inspiredpencil.com
Osmolality How To Find Osmolality Of Solution in simpler terms, osmolality is an expression of solute osmotic concentration per mass of solvent, whereas osmolarity is per volume. to calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. An osmole is 1 mol of. the osmolality of a real solution corresponds to the molality of an. How To Find Osmolality Of Solution.
From www.chegg.com
Solved Calculate the osmolarity of the following solutions. How To Find Osmolality Of Solution in simpler terms, osmolality is an expression of solute osmotic concentration per mass of solvent, whereas osmolarity is per volume. to calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. Convert that number to osmoles per liter and add. An osmole is 1 mol of. Osmolarity is the number. How To Find Osmolality Of Solution.
From www.youtube.com
Calculated osmolality and osmolality gap YouTube How To Find Osmolality Of Solution to calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. An osmole is 1 mol of. you multiply the molarity by the number of osmoles that each solute produces. in simpler terms, osmolality is an expression of solute osmotic concentration per mass of solvent, whereas osmolarity is per. How To Find Osmolality Of Solution.
From www.chegg.com
Solved Concentration Osmolarity . Ca 2.12.8 How To Find Osmolality Of Solution you multiply the molarity by the number of osmoles that each solute produces. how do you calculate osmolarity? in simpler terms, osmolality is an expression of solute osmotic concentration per mass of solvent, whereas osmolarity is per volume. An osmole is 1 mol of. the osmolality of a real solution corresponds to the molality of an. How To Find Osmolality Of Solution.
From www.coursehero.com
[Solved] I need help figuring out how to calculate molality and How To Find Osmolality Of Solution Convert that number to osmoles per liter and add. the osmolality of a real solution corresponds to the molality of an ideal solution containing nondissociating solutes and is expressed. osmolarity is a measure of the total concentration of solute particles in a solution, influencing the movement of. Osmolarity is the number of osmoles of solute per litre of. How To Find Osmolality Of Solution.
From www.youtube.com
Calculated Osmolality YouTube How To Find Osmolality Of Solution An osmole is 1 mol of. osmolarity is a measure of the total concentration of solute particles in a solution, influencing the movement of. the osmolality of a real solution corresponds to the molality of an ideal solution containing nondissociating solutes and is expressed. you multiply the molarity by the number of osmoles that each solute produces.. How To Find Osmolality Of Solution.
From www.youtube.com
Osmolarity Example (Bio) YouTube How To Find Osmolality Of Solution An osmole is 1 mol of. the osmolality of a real solution corresponds to the molality of an ideal solution containing nondissociating solutes and is expressed. in simpler terms, osmolality is an expression of solute osmotic concentration per mass of solvent, whereas osmolarity is per volume. Osmolarity is the number of osmoles of solute per litre of solution.. How To Find Osmolality Of Solution.
From www.reddit.com
How to calculate the osmolality of 0.1 M solution of Na2HPO4 + NaH2PO4 How To Find Osmolality Of Solution The osmolarity of solutions containing a single type of solute (for example: An osmole is 1 mol of. the osmolality of a real solution corresponds to the molality of an ideal solution containing nondissociating solutes and is expressed. osmolarity is a measure of the total concentration of solute particles in a solution, influencing the movement of. Osmolarity is. How To Find Osmolality Of Solution.
From ojklmexhbr.blogspot.com
How To Calculate Serum Osmolality In this study, we compared the How To Find Osmolality Of Solution An osmole is 1 mol of. how do you calculate osmolarity? the osmolality of a real solution corresponds to the molality of an ideal solution containing nondissociating solutes and is expressed. Osmolarity is the number of osmoles of solute per litre of solution. osmolarity is a measure of the total concentration of solute particles in a solution,. How To Find Osmolality Of Solution.
From www.coursehero.com
[Solved] I need help figuring out how to calculate molality and How To Find Osmolality Of Solution you multiply the molarity by the number of osmoles that each solute produces. The osmolarity of solutions containing a single type of solute (for example: to calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. osmolarity is a measure of the total concentration of solute particles in a. How To Find Osmolality Of Solution.
From exonhkusy.blob.core.windows.net
How To Calculate Osmolality Of Glucose at Robert Villegas blog How To Find Osmolality Of Solution in simpler terms, osmolality is an expression of solute osmotic concentration per mass of solvent, whereas osmolarity is per volume. how do you calculate osmolarity? osmolarity is a measure of the total concentration of solute particles in a solution, influencing the movement of. the osmolality of a real solution corresponds to the molality of an ideal. How To Find Osmolality Of Solution.
From www.numerade.com
SOLVED Calculate the Osmolarity and Osmolality of NaCl in 0.9 (w/v How To Find Osmolality Of Solution Convert that number to osmoles per liter and add. The osmolarity of solutions containing a single type of solute (for example: Osmolarity is the number of osmoles of solute per litre of solution. osmolarity is a measure of the total concentration of solute particles in a solution, influencing the movement of. An osmole is 1 mol of. how. How To Find Osmolality Of Solution.
From www.coursehero.com
[Solved] I need help figuring out how to calculate molality and How To Find Osmolality Of Solution The osmolarity of solutions containing a single type of solute (for example: An osmole is 1 mol of. to calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. how do you calculate osmolarity? Osmolarity is the number of osmoles of solute per litre of solution. in simpler terms,. How To Find Osmolality Of Solution.
From www.youtube.com
How to solve osmolarity calculation problems 4 YouTube How To Find Osmolality Of Solution to calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. An osmole is 1 mol of. osmolarity is a measure of the total concentration of solute particles in a solution, influencing the movement of. Osmolarity is the number of osmoles of solute per litre of solution. you multiply. How To Find Osmolality Of Solution.
From www.youtube.com
Calculating the Osmolarity of a Solution (Question 2) YouTube How To Find Osmolality Of Solution osmolarity is a measure of the total concentration of solute particles in a solution, influencing the movement of. the osmolality of a real solution corresponds to the molality of an ideal solution containing nondissociating solutes and is expressed. how do you calculate osmolarity? Osmolarity is the number of osmoles of solute per litre of solution. in. How To Find Osmolality Of Solution.
From www.youtube.com
Calculate your own osmolarity Lab values and concentrations Health How To Find Osmolality Of Solution you multiply the molarity by the number of osmoles that each solute produces. osmolarity is a measure of the total concentration of solute particles in a solution, influencing the movement of. Osmolarity is the number of osmoles of solute per litre of solution. Convert that number to osmoles per liter and add. The osmolarity of solutions containing a. How To Find Osmolality Of Solution.